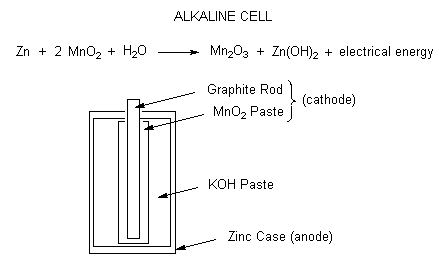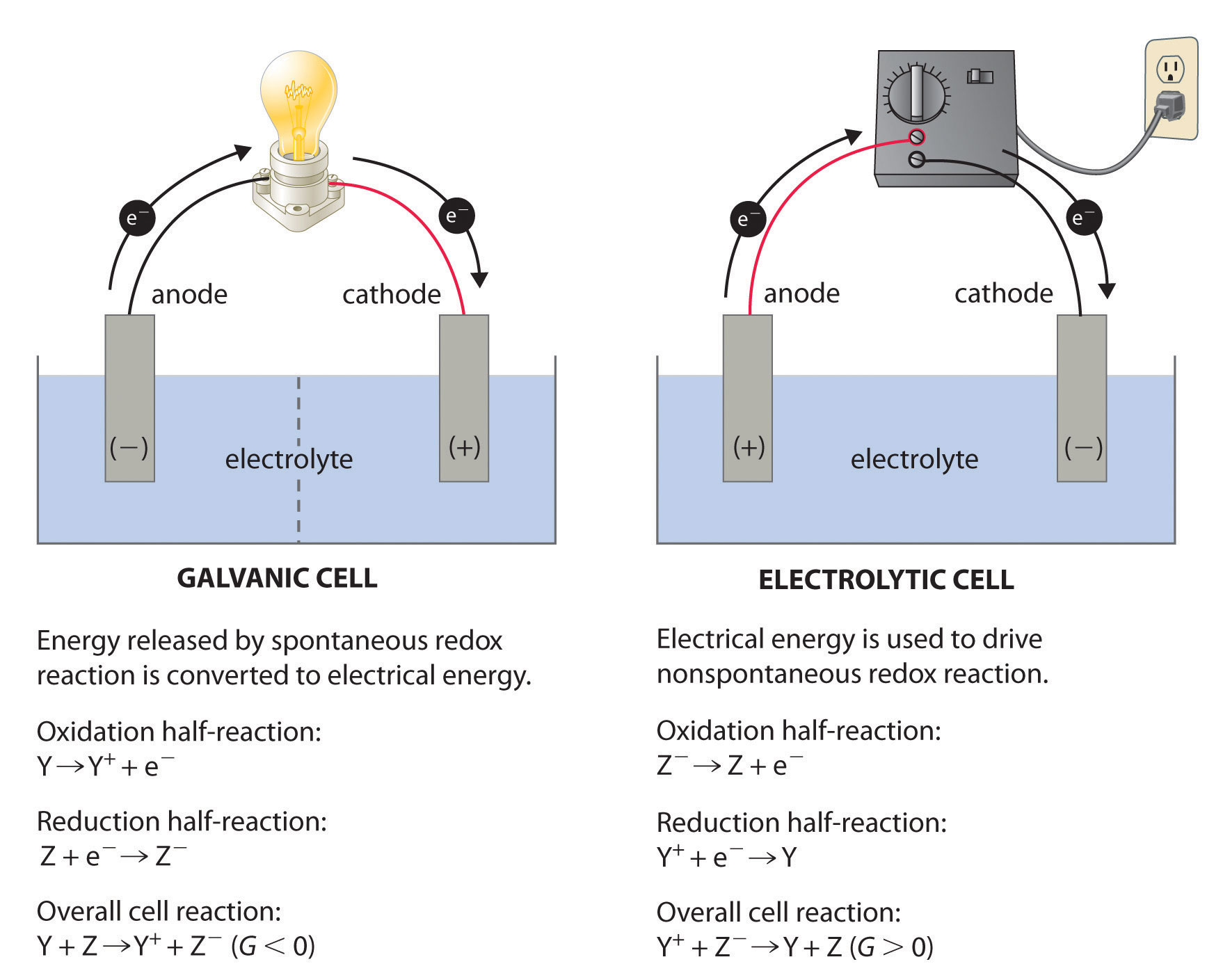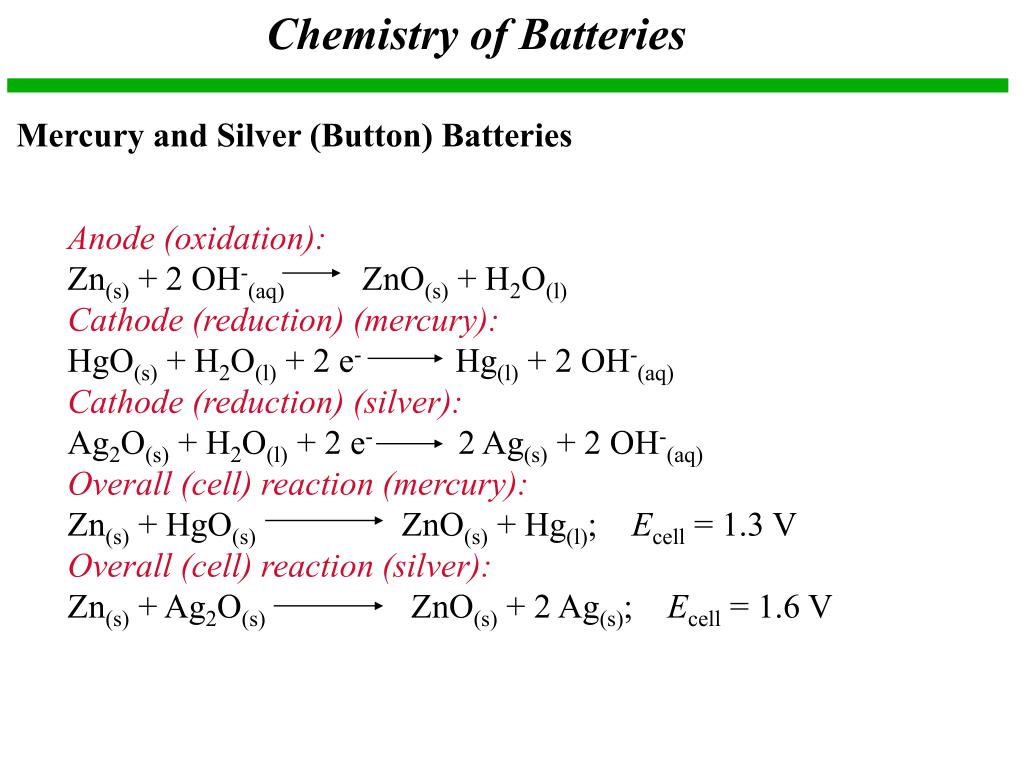
PPT - Chapter 21: Electrochemistry Chemical Change and Electrical Work PowerPoint Presentation - ID:3345926
Write the anode and cathode reactions occurring in a commonly used mercury cell. How is the overall reaction represented? - Sarthaks eConnect | Largest Online Education Community

Question Video: Calculating Electrode Potential When Given a Cell Potential and the Other Electrode Potential | Nagwa

Tony your fa ? Write anode and cathode reactions that occur in dry cell. How does a dry cell differ from a mercury cell ?

Mercury Cell process flow diagram (reproduced with permission of Euro... | Download Scientific Diagram

Anode vs. Cathode in Electrochemical Cells | Reaction & Notation - Video & Lesson Transcript | Study.com

class 12- chapter 3||Q. Why button cell / mercury cell produce constant current during its lifetime? - YouTube

write the reactions occurring at anode and cathode during working of a Mercury cell why does a voltage of a - Chemistry - Electrochemistry - 16289389 | Meritnation.com

What is the Difference Between Mercury Cell and Diaphragm Cell | Compare the Difference Between Similar Terms




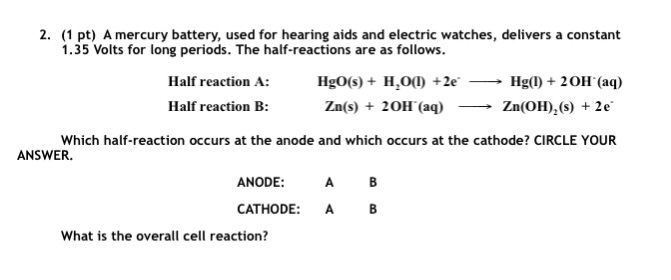

![Class 12] a. Why does cell voltage of a mercury cell remain constant Class 12] a. Why does cell voltage of a mercury cell remain constant](https://d1avenlh0i1xmr.cloudfront.net/4a474cf9-e1fe-4c2d-8b54-1c2705a88e9c/question-33-choice-a---why-does-the-cell-voltage-of-a-mercury-cell-remain---teachoo.jpg)